AMYOTROPHIC LATERAL SCLEROSIS
Using Angiogenesis to Treat Amyotrophic Lateral Sclerosis (ALS)
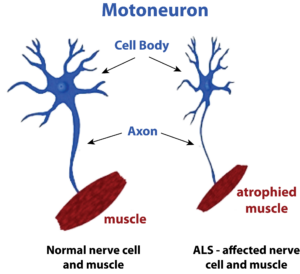
Wund is advancing the hypothesis that neurodegenerative diseases, such as Amyotrophic Lateral Sclerosis (ALS), are initiated and progress due to a lack of blood flow in the brains of individuals suffering from these neurological disorders. This lack of adequate blood flow gradually starves neurons in different areas of the brain leading to dysfunctional neurons and eventually death of the neurons due to a lack of nourishment and oxygen, as well as improper removal of metabolic wastes from the neurons. ALS is a neurodegenerative disease characterized by the selective loss of motor neurons in the brain and spinal cord. A review of the medical literature supports the idea that a chronic lack of blood flow to these motor neurons may underlie the development and progression of ALS and that therapeutic angiogenesis which can be induced by our drug, FGF-1, could increase blood perfusion in the brain, and possibly be a treatment for ALS.
Evidence of a Blood Perfusion Defect in ALS
ALS is a severe neurodegenerative disease with a complicated pathogenesis. Compelling evidence indicates impairment of the neurovascular unit components in motor neurons that are affected in this disease. In addition, defects in the blood-brain and blood-spinal cord barriers in both human and animal models, have led to the classification of ALS as a neurovascular disease.
Motor neurons have extremely high metabolic demands, and so it follows that they might be unusually sensitive to a mild decrease in the blood supply. Not only are they among the largest cells in the body (their volume is up to 5,000 times that of typical cells), but they can be very long (up to a meter in length in humans), necessitating the transport of proteins and other macromolecules across great distances.
Added to this, motor neurons maintain a high rate of electrical firing, placing an enormous demand for an energy supply to maintain this electrical activity. Thus, chronically reduced vascular perfusion could produce deficits in oxygen and glucose, and so fail to meet the energy requirements of motor neurons. The diminishment of vascular perfusion rates, along with age, may contribute to the selective vulnerability of motor neurons to a lack of blood flow, and along with other genetic and environmental factors, may be a triggering event for ALS.
Studies in Animal Models of ALS
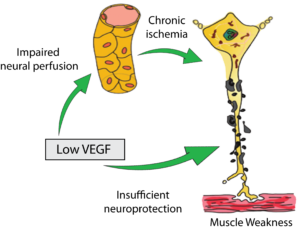
In ground-breaking research, scientists from Duke University and UC, San Diego showed that the lack of the growth factor, VEGF, in mice produced all the classical features of ALS. The lack of VEGF produced degeneration of motor axons and the characteristic denervation-induced muscle atrophy. As in ALS, the neuronal deficits were selective for motor neurons—sensory neurons and neurons in other brainstem nuclei were not affected. In addition, the scientists also established that the ALS symptoms developed not only from a lack of blood perfusion, but also due to a lack of neuroprotection of the motor neurons due to this lack of VEGF. Figure 2 characterizes the main findings of this research. This figure depicts that low levels of the angiogenic growth factor, VEGF, results in impaired blood perfusion due to a lack of angiogenesis, resulting in chronic ischemia, motor neuron degeneration and muscle weakness. In addition, insufficient neuroprotection could also lead to motor neuron degeneration.
It is known that FGF-1 sits above VEGF in the angiogenesis cascade and that FGF-1 is a potent stimulator of VEGF production and release in ischemic tissues. FGF-1 is also neuroprotective for motor neurons and so should be able to completely replicate all of these earlier findings seen with VEGF in mice.
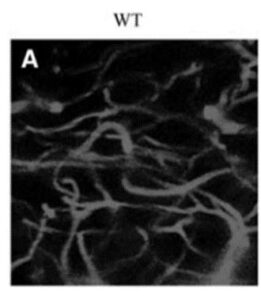
In another important study, scientists have also been examining the early and progressive impairment of spinal blood flow in motor neuron degeneration seen in ALS mice. In that study, the scientists performed capillary imaging in mice and directly measured spinal blood flow and glucose metabolism in their spinal cords. The capillary imaging showed a progressive decrease of capillary diameter, capillary density, and spinal blood flow during the disease course (from the pre-symptomatic stage to the end stage of the disease). It was established that there was a strong correlation between decreasing capillary diameter and density which led to the disruption of the “neuro-vascular unit” in the regions of the brain and spinal cord affected by ALS. This can be seen clearly in the Figure 3 where capillaries in similar regions of the spinal cord were imaged in live animals, without ALS (A) and with ALS (D).
There is a significant decrease in the diameter and density of capillaries seen in the ALS mouse (D), which would be expected to contribute to the motor neuron degeneration and muscle weakness seen in these animals. The researchers went on to conclude that this vascular pathology could be profoundly involved in the whole disease process of ALS and represents a potential target for therapeutic intervention of ALS. We believe it represents an excellent therapeutic target to study in human subjects with ALS, subjects who will be given IV infusions of the FGF-1 molecule over a period of months.
Studies in Patients with ALS
Turning to research in human subjects with ALS, sensitive brain imaging technology has been used to attempt to match ALS severity with the level of blood flow in the brain (5). Medical researchers have analyzed brain blood flow in the brains of ALS patients by using an MRI method referred to as “arterial spin labeling”, a non-invasive form of magnetic resonance imaging.
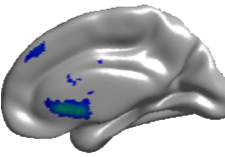
A total of 55 ALS patients and 20 healthy controls (HCs) were evaluated for patterns of brain atrophy and perfusion as measured by arterial spin labeling (ASL)-MRI. The research has shown a strong correlation between decreased blood perfusion in the cerebral cortex and the development of dementia in patients with ALS. The researchers utilized the MRI technique of arterial spin labeling to characterize the patterns of brain atrophy and blood perfusion in ALS patients with varying levels of cognitive deficit, including ALS with frontotemporal dementia (FTD). They established that the more severe the lack of blood perfusion was in certain areas of the cortex, the more profound was the patients’ dementia. This can be seen in Figure 4 where blood flow in the cortex is measured in real time by MRI in ALS patients with and without dementia. The areas of diminished blood flow are represented by blue and green regions and it can clearly be seen that the areas of decreased blood flow in the patient with dementia cover a much broader area of the cortex, areas involved in cognition and executive functioning.

Figure 4. Brains of ALS Patients with (top) and without (bottom) Dementia
Conclusion
There is a large and accumulating body of evidence that vascular dysfunction and diminished blood flow in the brain is an initiating factor in the development of neurodegenerative diseases.
There is ample evidence in both animal and human studies that a lack of blood perfusion to brain and spinal cord motor neurons is a critical event in the initiation of ALS. We believe that the slow and inexorable decline of angiogenesis and neurogenesis occurs in the brains of ALS patients and leads to disease progression. A growth factor which can potently stimulate those two physiological processes is an attractive candidate to test in clinical trials. Human FGF-1 is such a candidate. It is a natural growth factor in our bodies that is constantly put to use to heal and regenerate injured tissues or organs.
We hope within 12 months to begin our testing of FGF-1 in clinical trials with ALS patients.
Research Papers and additional reading:
1. Hypoxia and Lou Gehrig. P Skene, D Cleveland. (2001) Nature Genetics 28:107-108. (Link: https://www.nature.com/articles/ng0601_107 )
2. Deletion of the hypoxia-response element in the vascular endothelial growth factor promoter causes motor neuron degeneration. Oosthuye, B. et al. (2001).Nature Genetics 131–138. https://www.ncbi.nlm.nih.gov/pubmed/11381259
3. Fibroblast Growth Factor-1 Induces Heme Oxygenase-1 via Nuclear Factor Erythroid 2-related Factor 2 (Nrf2) in Spinal Cord Astrocytes. Vargas MR et al. J (2005). Biol Chem 280:25571-25578. https://www.ncbi.nlm.nih.gov/pubmed/15870071
4. Early and progressive impairment of spinal blood flow–glucose metabolism coupling in motor neuron degeneration of ALS model mice. Miyazaki K et al. J (2012) Cerebral Blood Flow Metab 32:456-467. https://www.ncbi.nlm.nih.gov/pubmed/22068226
5. Gray matter perfusion correlates with disease severity in ALS. Rule RR et al. (2010). Neurology 74:821-827. https://www.ncbi.nlm.nih.gov/pubmed/20147656
6. Brain structural and perfusion signature of amyotrophic lateral sclerosis with varying levels of cognitive deficit. Shen D et al. (2018). Frontiers in Neurology 9:1-9. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5976730/
7. Acidic fibroblast growth factor (FGF-1) in the anterior horn cells of ALS and control cases. Kage M, Yang Q, Sato H, Matsumoto S, Kaji R, Akiguchi I, Kimura H, Tooyama I. (2001) Neuroreport. 2001 Dec 4;12(17):3799-803. https://www.ncbi.nlm.nih.gov/pubmed/11726798