TRAUMATIC BRAIN INJURY
Treating Traumatic Brain Injuries (TBI) with Angiogenic Growth Factors
Traumatic brain injury (TBI) usually results from a violent blow or jolt or blast or bullet to the head. We believe that this trauma damages the microvasculature which causes a slow cascade of problems that become worse over time. We believe that based on evidence, we can use growth factors to repair this damage to the microvasculature and to the neurons.
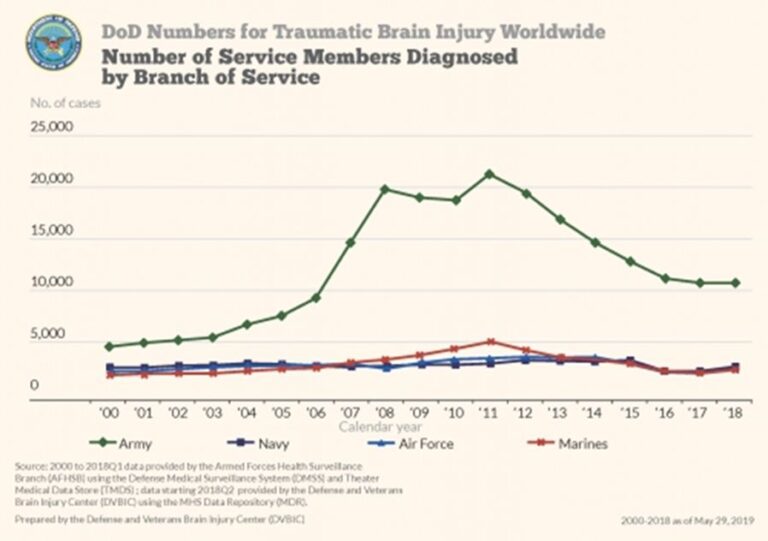
TBI is prevalent in the United States. In military settings, blast exposure is the leading cause of TBI. For U.S. forces deployed to Afghanistan and Iraq estimates of the prevalence of TBI among returning service members range from 15.2% to 22.8%, affecting as many as 320,000 troops. As shown in Figure 1, there has been a precipitous rise in reported cases of military TBI.
Mild traumatic brain injury may affect your brain cells temporarily. More serious traumatic brain injury can result in bruising, torn tissues, bleeding and other physical damage to the brain. These injuries can result in long-term complications or death.
Traumatic brain injury can have wide-ranging physical and psychological effects. Some signs or symptoms may appear immediately after the traumatic event, while others may appear days or weeks later. Despite their frequency, the acute and long-term effects of TBI have been a relatively unexplored area of medical inquiry until very recently. Undoubtedly, the “invisible” nature of many TBIs, notably the lack of any external physical evidence of damage to the head or brain, has been a major factor contributing to the impression that these soldiers have an “inconsequential” injury. However, there is good evidence that many individuals develop persistent cognitive and behavioral changes, even after mild neurotrauma.
Understanding the effects of military-related TBI presents additional challenges not encountered in studies of neurotrauma seen in athletes or other at-risk groups. With sports-related brain trauma, the injury is generally dependent on the rules of engagement specific to the sport. With military-related TBI, trauma can occur in widely heterogeneous ways, including recreational activities, physical training practices, falls, motor-vehicle accidents, and exposure to explosive blasts. Injury from explosive blasts varies depending on the strength of the explosive and whether the injury occurs in an open field, near buildings or in a motor vehicle. Military TBI is also random and unpredictable, ranging from a single injury to many thousands of traumatic injuries over similar time periods depending on an individual's exposure to blasts and impacts.
Traumatic Brain Injury (TBI) Damages the Brain’s Microvasculature
The pathology behind traumatic brain injuries has been well studied and multiple animal models of TBI have been developed to examine the pathophysiology of this type of trauma. In general, rapid acceleration, deceleration, or rotational forces cause the brain to elongate and deform, stretching individual cells and blood vessels and altering membrane permeability. Although all cell compartments are affected by the injury, axons are especially vulnerable to shear injury given their relatively long length.
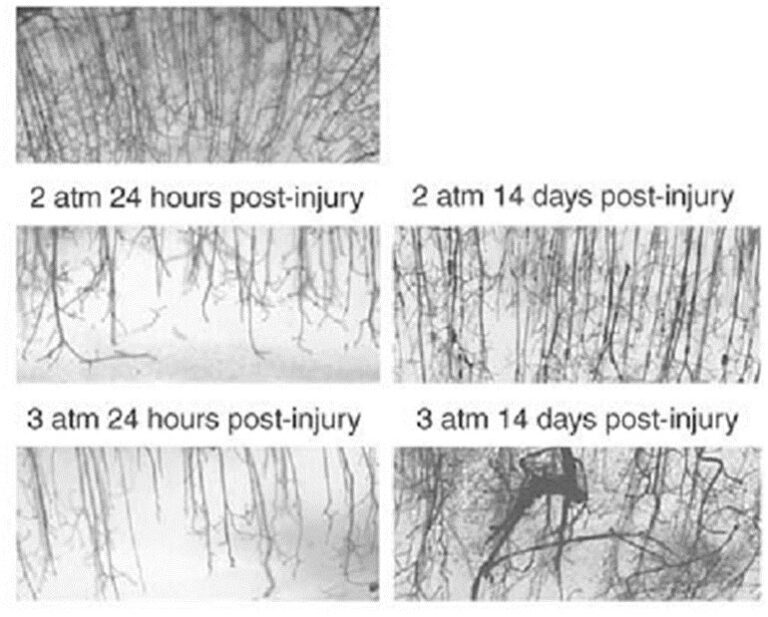
On the top panel of Figure 2, a dense and well-ordered microvasculature can be seen in a control animal with a sham (fake) TBI. In animals injured with 2 atmospheres (middle panels) and 3 atmospheres (bottom panels) of pressure, depletion of the microvasculature is readily apparent at 24 hours post-injury injury. Upon recovery at 14 days the microvascular has been reestablished but it is less dense and more disordered than in the control animals. There is a significant depletion of the microvasculature at 24 hours post-injury and the relatively disordered re-growth of the microvasculature at 14 days post-injury.
From the above results, it could be anticipated that neurons would most likely be under-perfused in those areas of recovery which display a disordered microvasculature. This condition has been termed traumatic cerebral vascular injury and is thought to not only underlie the many deficits seen in TBI, but also in its chronic form, to actively participate in the pathogenesis of neurogenerative diseases including Alzheimer’s disease and Parkinson’s disease.
Conclusion
Wund believes that FGF-1 can benefit patients with debilitating neurodegenerative diseases, including TBI and Major Depressive Disorder. These diseases are unmet medical needs and we firmly believe that there is a strong body of evidence indicating that these degenerative diseases have as a primary initiating cause, dysfunction in the brain’s microvasculature. We believe that FGF-1, as a stimulator of angiogenesis in the microvasculature, could be given early in these brain disorders, to potentially prevent the downward spiral to neuron cell injury and death. Even if given later in the disease progression, we believe that FGF-1, with its ability to co-regulate both angiogenesis and neurogenesis, could also be of possible benefit in later stages of the disease process.
Research Papers and additional reading:
1. The FGF family: biology, pathophysiology and therapy. Beenken A and Mohammadi M. Nat Rev Drug Discov 2009; 8: 235-253. https://www.ncbi.nlm.nih.gov/pubmed/19247306
2. Quantitative Changes in Hippocampal Microvasculature of Chronically Stressed Rats: No Effect of Fluoxetine Treatment. Czeh B et al. Hippocampus 2010; 20:174–185. https://www.ncbi.nlm.nih.gov/pubmed/19330847
3. Neurogenesis in the adult human hippocampus. Eriksson et al. Nature Medicine 1998; 4: 1313-1317. https://www.ncbi.nlm.nih.gov/pubmed/9809557
4. Fibroblast growth factors and their receptors. Gaizie Z, Kinsella AR, Smith JA. Biochem Cell Biol 1997; 75:669-685. https://www.ncbi.nlm.nih.gov/pubmed/9599656
5. Alzheimer’s Disease Neuroimaging Initiative. Early role of vascular dysregulation on late-onset Alzheimer’s disease based on multifactorial data-driven analysis. Iturria-Medina Y, R.C. Sotero, P.J. Toussaint, J.M. Mateos-Perez, A.C. Evans & The Nature Communications 2016, 7:1-14. https://www.nature.com/articles/ncomms11934
6. Cerebral Vascular Injury in Traumatic Brain Injury. Kenney K et al. Experimental Neurology 2016; 275:353-366. https://www.sciencedirect.com/science/article/abs/pii/S0014488615300066
7. Military-related traumatic brain injury and neurodegeneration. McKee AC and Robinson ME. Alzheimer’s Dement. 2014; 10: S242–S253. https://www.ncbi.nlm.nih.gov/pubmed/24924675
8. The spectrum of disease in chronic traumatic encephalopathy. McKee AC et al. Brain 2013; 136:43-64. https://www.ncbi.nlm.nih.gov/pubmed/23208308
9. Traumatic brain injury results in acute rarefication of the vascular network. Obenaus A et al. Nature Scientific Reports 2017:239. https://www.ncbi.nlm.nih.gov/pubmed/28331228
10. An analysis of regional microvascular loss and recovery following two grades of fluid percussion trauma: a role for hypoxia-inducible factors in traumatic brain injury. Park E et al. J Cereb Blood Flow Metab 2009; 29:575-584. https://journals.sagepub.com/doi/10.1038/jcbfm.2008.151
11. Induction of Neoangiogenesis in Ischemic Myocardium by Human Growth Factors. First Clinical Results of a New Treatment of Coronary Heart Disease. Schumacher B, P Pecher, BU von Specht, TJ Stegmann: Circulation 1998; 97:645-650. https://www.ahajournals.org/doi/10.1161/01.CIR.97.7.645
12. Thomas K, Rios-Candelore M, Gimenez-Gallego G, et al. Proc Natl Acad Sci USA. 1985; 82:6409-13. https://www.ncbi.nlm.nih.gov/pubmed/2413439
13. The War Within: Preventing Suicide in the US Military. Tanielian T and Jaycox L (editors). RAND Corporation, 2011. https://www.rand.org/content/dam/rand/pubs/monographs/2011/RAND_MG953.pdf
14. Midlife vascular risk factors and Alzheimer’s disease: evidence from epidemiological studies. Tolppanen AM, Solomon A, Soininen H, Kivipelto M. J Alzheimer’s Dis 2012; 32: 531–40. https://www.ncbi.nlm.nih.gov/pubmed/22842867
15. The Fibroblast Growth Factor Family: Neuromodulation of affective behavior. Turner CA, Watson SJ, Akil H: Neuron 2012, 76:160-174. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3476848/
16. Dysregulation of the fibroblast growth factor system in major depression. S. J. Evans, et al. Proc Natl Acad Sci U S A. 2004 Oct 26; 101(43): 15506–15511. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC523463/