CHRONIC TRAUMATIC ENCEPHALOPATHY
Treating Chronic Traumatic Encephalopathy (CTE) with Angiogenic Growth Factors
Wund believes that Chronic Traumatic Encephalopathy (CTE) is microvascular dysfunction of the brain, caused by repetitive blows to the brain, that can be treated by angiogenic growth factors. CTE is a unique neurodegenerative condition that is associated with repetitive mild traumatic brain injuries which occur in a wide variety of contact sports, including football, boxing, wrestling, rugby, hockey, lacrosse, soccer, and skiing. In collision sports, such as football (Omalu et al, 2005) and boxing (Corsellis et al 1973), players may experience thousands of sub-concussive hits over the course of a single season. Furthermore, combat military veterans is another large group of individuals prone to repetitive head trauma, and are at risk for CTE.
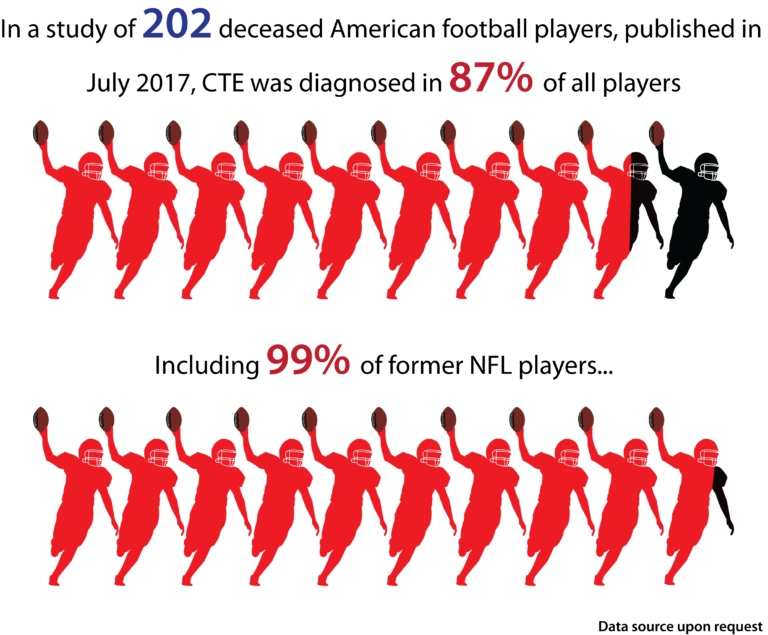
Most sports-related head injuries are minor and although the majority of athletes who suffer a concussion, recover within a few days or weeks, a number of these individuals will go on to develop long-lasting or progressive symptoms. This is especially true in cases of repetitive concussion or repetitive mild traumatic brain injury in which at least 17% of individuals develop encephalopathy (CTE), and this number may be an underestimation of the problem. It is unclear what severity or recurrence of head injury is required to initiate CTE; no well-designed prospective studies have addressed these important issues. The severity of the disorder appears to correlate with the length of time engaged in the sport and the number of traumatic injuries, although whether a single traumatic brain injury can trigger the onset of CTE remains a matter of speculation.
CTE was originally reported in 1928 by a New Jersey pathologist (Martland, 1928) who described the clinical aspects of a progressive neurological deterioration (“punch drunk”) that occurred after repetitive brain trauma in boxers. CTE is associated with symptoms of irritability, impulsivity, aggression, depression, short-term memory loss and heightened suicidality that usually begin 8–10 years after experiencing repetitive mild traumatic brain injury. With advancing disease, more severe neurological changes develop that include dementia, gait and speech abnormalities and symptoms resembling Parkinson’s disease. In late stages, CTE may be clinically mistaken for Alzheimer’s disease or vascular dementia.
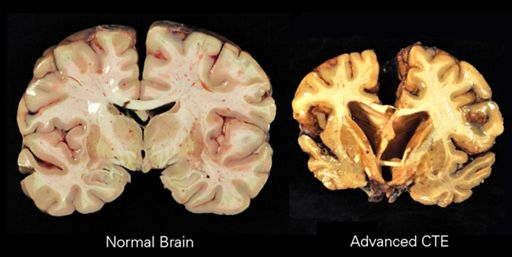
The evidence suggests that CTE begins at a defined focal area in the brain, usually adjacent to small blood vessels and in the cerebral cortex, an area of the brain that plays a key role in memory, attention, cognition and language. The disease spreads slowly over decades to involve widespread and diverse regions of the brain, including areas of the brain that control long-term memory, executive functioning and dopamine production. The neuropathological changes of CTE are distinctive and easily distinguished from other neurogenerative diseases, including Alzheimer’s disease. There is a distinctive pattern of neurofibrillary tangles (composed of phosphorylated-tau proteins) seen in CTE, which differs from Alzheimer’s disease. In addition, β-Amyloid (Aβ) deposition, which is a hallmark of Alzheimer’s disease is not consistently seen in CTE. Over time neuronal loss becomes substantial in CTE and can result in a significant decrease in brain volume as is apparent in Figure 1.
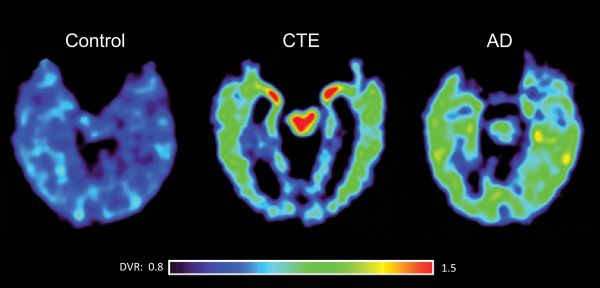
Presently there are no available biomarkers for the diagnosis of CTE. However, advances in neuroimaging, most notably the development of PET scanning procedures that can image tau containing neurofibrillary tangles, offer the promise of detecting CTE in its earlier stages. As shown in Figure 2, PET scanning with an investigational fluorescent tracer (termed FDDNP) which binds specifically to the tau protein in neurofibrillary tangles may reveal chronic traumatic encephalopathy (CTE) in living athletes and differentiate it from Alzheimer's disease. The scan at the far right of a patient with Alzheimer’s disease shows a different distribution of the tau tracer. Red and yellow areas indicate high FDDNP binding signals (Barrio et al, 2013).
Microvascular Dysfunction in Chronic Traumatic Encephalopathy
Recent research has indicated that for two neurogenerative diseases, Alzheimer’s disease and Parkinson’s disease, a critical early and possible precipitating event is dysfunction in the microvasculature leading to a lack of blood flow and a diminished capacity of the brain neurons. In probably the largest study of its kind, researchers from McGill University in Montreal recently completed a study in which they followed disease progression in 1,171 patients with Alzheimer’s disease. They developed a 30-year trajectory for biomarkers of Alzheimer’s disease and reported that the very first changes seen in the disease was a deterioration of the microvasculature leading to under-perfusion of brain neurons. These vasculature changes occurred before any of the symptomatic changes in Alzheimer disease occurred, including structural changes in the brain, accumulation of beta amyloid plaques or cognitive decline.
With Parkinson’s disease, research being conducted at the University of Southern California has recently led to the hypothesis that micro-vascular dysfunction in the brain and flaws in the blood/brain barrier may lead to the damage and ultimate destruction of selected population of neurons, including the dopamine-producing cells that are depleted in Parkinson’s disease.
There is the evidence that disrupted blood perfusion in the brain has a link to the initiation and progression of CTE. There are several lines of evidence that point to a strong link between vascular dysfunction and CTE. In human brain specimens taken at autopsy from CTE victims, microvascular disruption is seen almost universal. Prominent cerebral microvasculature pathology was reported in some of the first cases of CTE described by Corsellis in 1973. Extensive microvascular dysfunction was also reported in active duty military service members with CTE who had been exposed to multiple blast explosions (Goldstein 2012, McKee 2013, 2014). Finally, striking vascular changes have been seen in specimens from sports-related cases of CTE (McKee 2014).
To date, no studies have examined in living subjects the extent of microvasculature damage in the early stages of CTE. However, there is very strong evidence of a link between underperfusion of brain neurons and subsequent impairments from medical research into the pathophysiology of traumatic brain injury (TBI). Both in animal models of TBI and in patients with TBI, direct and long-lasting damage to the microvasculature of the brain can be demonstrated (Kenney 2016).
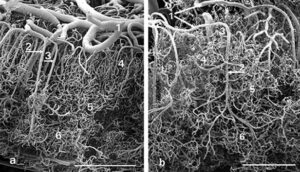
As seen in Figure 3, scanning electron microscopy from a patient with TBI indicates a remarkable disordering of the cerebral vasculature. The left panel shows the microvasculature from a normal area of the brain while the right panel shows a micrograph from a damaged area of the brain (from Rodriquez-Baeza, 2003). From Figure 3, it is readily apparent that with TBI there is a collapsing and flattening of small arteries in the microvasculature (areas labelled #1 in both panels) as well as a gross disordering of the smaller capillaries which would nourish adjacent neurons (areas labelled #4 in both panels).
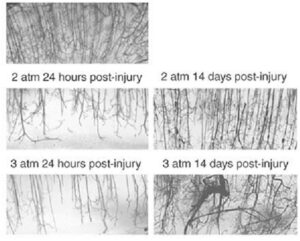
Striking microvascular disordering has also been consistently observed in animal models of TBI. As shown in Figure 4, rodents that have been subjected to experimental TBI and then allowed to recover showed a microvasculature that is less dense and more disordered. In these experiments, rodents were subjected to pressure-induced fluid percussion TBI, allowed to recover and then the brain microvasculature was examined 24 hours and 14 days post-injury (from Park, 2009).
On the top panel of Figure 4, a dense and well-ordered microvasculature can be seen in a control animal with a sham (fake) TBI. In animals injured with 2 atmospheres (middle panels) and 3 atmospheres (bottom panels) of pressure, depletion of the microvasculature is readily apparent at 24 hours post-injury injury. Upon recovery at 14 days the microvascular has been reestablished but it is less dense and more disordered than in the control animals.
From the above results, it could be anticipated that neurons would most likely be under-perfused in those areas of recovery which display a disordered microvasculature. In addition, it is also not hard to imagine that if this trauma was repeated continuously the accumulated microvasculature damage would lead to diminished neuronal functioning and ultimately, nerve cell death, a condition that is consistent with chronic traumatic encephalopathy.
Angiogenesis – A Treatment Modality for CTE That Should Be Examined
Angiogenesis is a natural regenerative process that the body uses to produce new blood vessels in damaged tissues or organs. Angiogenesis is controlled by a number of growth factors and cytokines and these factors (and their cell surface receptors) are upregulated in response to injury or ischemia. Probably one of the most potent known stimulators of angiogenesis is the human protein, FGF-1. This growth factor is part of a large family (22 members) of proteins termed fibroblast growth factors. FGF-1 is the only member of this family that can activate all 7 cell surface receptors that mediate the biological activities of the FGF family, making it a growth factor that has highly diverse activities in the body. FGF-1 also is a potent inducer of the production of other cytokines that are involved in tissue repair and regeneration, setting off a cascade of metabolic activities critical for tissue repair and healing.
Therapeutic angiogenesis is the use of angiogenic agents such as FGF-1 to stimulate new blood vessel formation in tissues and organs damaged by a lack of blood perfusion. FGF-1 has enjoyed remarkable clinical success in a number of USA FDA-authorized clinical trials, including severe coronary artery disease and diabetic foot ulcers, with both disorders a result of poor blood flow.
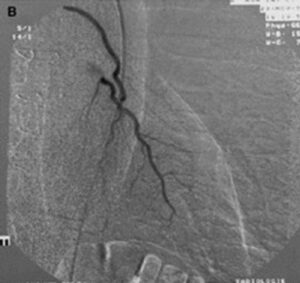
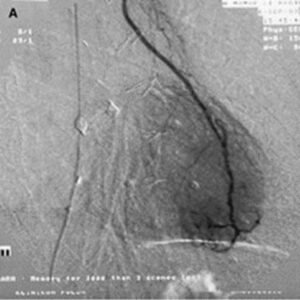
FGF-1 was in Phase II clinical trials before its clinical development was suspended due to financial reasons (and not for clinical efficacy or safety reasons). Figure 5 shows the characteristic “blush” of newly formed blood vessels that is seen when FGF-1 is injected into the hearts of patients with coronary artery disease.
In a series of human trials FGF-1 was shown to be a robust inducer of angiogenesis in patients with coronary artery disease with new vessels that were still functioning 3 years after treatment and with patients enjoying a significant improvement in clinical markers including exercise performance and angina scores as well quality of life indexes. Given our contention that a disruption of the microvasculature in the brain is a contributing or sole factor in the development of CTE, clinical studies with FGF-1 in CTE patients could yield promising results.
FGF-1 is Neuroprotective and Neurotrophic
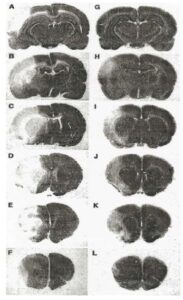
As mentioned above, FGF-1 has diverse biological activities and in addition to its angiogenesis properties, there is a large body of medical literature that has established that FGF-1 can not only protect neurons from harmful stimuli (such as hypoxia during stroke) but can also stimulate the production of neural stem cells and new neurons in the adult brain. Figure 6 below shows the neuroprotection that FGF-1 can provide in an animal model of stroke where IV administered FGF-1 resulted in mice that had a much smaller stroke infarcted area. These studies also demonstrate that FGF-1 can cross the blood brain barrier to gain access to areas of the brain affected by stroke.
In addition to its neuroprotective properties, FGF-1 has been shown in animal studies to stimulate neurogenesis, a process characterized by the development of new neuronal stem cells and their maturation into functioning nerve cells. Mice who had been given a permanent stroke lesion, followed by 4 weeks of FGF administration, developed an increased in brain axonal sprouting and subsequent enhanced recovery of motor function, including limb movement and coordination in mice. As CTE is characterized by damaged neurons and neurons at risk of dying, this additional neuroprotective activity of FGF-1 enhances its attractiveness to test as a possible therapy for this disease.
Conclusion
Based on recent research, Wund believes that that microvascular dysfunction is a critical early step in the progression of neurodegenerative diseases such as Alzheimer’s disease, Parkinson’s disease and CTE. A disruption in the small arteries supplying blood to selected neurons leads to a diminished functioning of the nerve cells. In autopsies of CTE victims and in animal models of CTE, researchers have also observed that repeated head trauma can lead to microvasculature damage in the brain, presumably leading to neurons with diminished capacity. Growth factors which can re-establish a normal microvasculature in the brain hold promise to treat these degenerative diseases.
Wund believes that our drug is an excellent candidate to test in clinical trials with CTE patients in an attempt to restore the brain’s microvasculature for the normal perfusion of brain neurons. Wund believes that its angiogenic and neurotrophic properties makes it a potential treatment for the treatment of CTE. The company plans to advance on setting up Clinical Protocols for trials in three patient populations, including individuals with late stage CTE, those with early-middle stage CTE and for prophylactic/preventative use in athletes who engage in high impact sporting events. Given both its angiogenic and neurogenic biological activities, Wund believes that FGF-1 is an extremely promising candidate to test in patients with CTE or those who are at risk to develop CTE.
Research Papers and additional reading:
1. In vivo characterization of chronic traumatic encephalopathy using [F-18] FDDNP PET brain imaging. Barrio JR, Small GW, Wong KP, Huang SC, Liu J, Merrill DA, Giza CC, Fitzsimmons RP,Omalu B, Bailes J, Kepe V.. Proc. Natl. Acad Sci USA. 2015; pii: 201409952: E2039- E2047. https://www.ncbi.nlm.nih.gov/pubmed/25848027
2. The aftermath of boxing. Corsellis JA, Bruton CJ, Freeman-Browne D.. Psychol Med 1973; 3:270– 303. https://www.cambridge.org/core/journals/psychological-medicine/article/aftermath-of-boxing1/4F0E256E19D4B8B06EE62AC517FAB853
3. Neurogenesis in the adult human hippocampus. Eriksson et al. Nature Medicine 1998; 4: 1313-1317. https://www.ncbi.nlm.nih.gov/pubmed/9809557
4. Fibroblast growth factors and their receptors. Gaizie Z, Kinsella AR, Smith JA. Biochem Cell Biol 1997; 75:669-685. https://www.ncbi.nlm.nih.gov/pubmed/9599656
5. Chronic traumatic encephalopathy in blast-exposed military veterans and a blast neurotrauma mouse model. Goldstein et al. Science Trans Med 2012; 4:1-7. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3739428/
6. Cerebral Vascular Injury in Traumatic Brain Injury. Kenney K et al. Experimental Neurology 2016; 275:353-366. https://www.sciencedirect.com/science/article/abs/pii/S0014488615300066
7. The spectrum of disease in chronic traumatic encephalopathy. McKee AC et al. Brain 2013; 136:43-64. https://www.ncbi.nlm.nih.gov/pubmed/23208308
8. The neuropathology of sport. McKee AC et al. Acta Neuropathology 2014; 127:29-51. https://www.ncbi.nlm.nih.gov/pubmed/24366527
9. Chronic traumatic encephalopathy in a National Football League player. Omalu BI, DeKosky ST, Minster RL, et al. Neurosurgery 2005; 57:128–34. https://www.ncbi.nlm.nih.gov/pubmed/15987548
10. Punch drunk. Martland HS. JAMA 1928;91:1103-1107. https://jamanetwork.com/journals/jama/article-abstract/260461
11. An analysis of regional microvascular loss and recovery following two grades of fluid percussion trauma: a role for hypoxia-inducible factors in traumatic brain injury. Park E et al. J Cereb Blood Flow Metab 2009; 29:575-584. https://www.semanticscholar.org/paper/An-Analysis-of-Regional-Microvascular-Loss-and-Two-Park-Bell/27e76c009ff6e56ead35e203cd60e6210e8c1696
12. PET scanning of brain tau in retired national football league players: preliminary findings. Small et al. Am J Geriatr Psychiatry 2013; 21:2. https://www.ncbi.nlm.nih.gov/pubmed/23343487
13. Induction of Neoangiogenesis in Ischemic Myocardium by Human Growth Factors. First Clinical Results of a New Treatment of Coronary Heart Disease. Schumacher B, P Pecher, BU von Specht, TJ Stegmann: Circulation 1998; 97:645-650. https://www.ahajournals.org/doi/full/10.1161/01.cir.97.7.645
14. Pure brain-derived acidic fibroblast growth factor is a potent angiogenic vascular endothelial cell mitogen with sequence homology to interleukin 1. Thomas K, Rios-Candelore M, Gimenez-Gallego G, et al. Proc Natl Acad Sci USA. 1985; 82:6409-13. https://www.pnas.org/content/82/19/6409
15. Midlife vascular risk factors and Alzheimer’s disease: evidence from epidemiological studies. Tolppanen AM, Solomon A, Soininen H, Kivipelto M. J Alzheimer’s Dis 2012; 32: 531–40. https://www.researchgate.net/publication/230582258_Midlife_Vascular_Risk_Factors_and_Alzheimer's_Disease_Evidence_from_Epidemiological_Studies
16. Alzheimer's disease: epidemiology, genetics, and beyond. Wang XP, Ding HL. Neurosci Bull. 2008; 24:105–109. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5552509/
17. FGF-1: a human growth factor in the induction of neoangiogenesis. Thomas J. Stegmann. Expert Opinion on Investigational Drugs. Volume 7, 1998 - Issue 12. https://www.tandfonline.com/doi/abs/10.1517/13543784.7.12.2011
18. Brain Imaging: Tackling Chronic Traumatic Encephalopathy. Dr. Francis Collins. NIH Directors’s Blog. April 21, 2015. https://directorsblog.nih.gov/2015/04/21/brain-imaging-tackling-chronic-traumatic-encephalopathy/
19. Morphological features in human cortical brain microvessels after head injury: A three‐dimensional and immunocytochemical study. Alfonso Rodríguez‐Baeza Francisco Reina‐de la Torre Antonia Poca Mercè Martí Angel Garnacho. American Association for Anatomy. May 28, 2003. https://anatomypubs.onlinelibrary.wiley.com/doi/full/10.1002/ar.a.10069
20. Early role of vascular dysregulation on late-onset Alzheimer’s disease based on multifactorial data-driven analysis. Y. Iturria-Medina, R. C. Sotero, P. J. Toussaint, J. M. Mateos-Pérez, A. C. Evans & The Alzheimer’s Disease Neuroimaging Initiative. Nature Communications volume 7, Article number: 11934 (2016). https://www.nature.com/articles/ncomms11934
21. Blood-Brain Barrier: From Physiology to Disease and Back. Melanie D. Sweeney, Zhen Zhao, Axel Montagne, Amy R. Nelson, and Berislav V. Zlokovic. Physiological Reviews. OCT 2018. https://journals.physiology.org/doi/full/10.1152/physrev.00050.2017