CHRONIC DEPRESSION
Chronic Depression: Treatable with Therapeutic Angiogenesis?
Depression is one of the most prevalent and life-threatening forms of mental illness, affecting about 21% of the world’s population and more than 20 million people in the United States. A more severe form of the disease, Major Depressive Disorder (MDD) and Chronic Depression (CD), affect more than 2 million Americans. CD is long-lasting (years) and improvements can be seen with anti-depressant medications and/or cognitive therapy, but inevitably, there is a relapse to a state of depression that has a severe and significant impact on the patient’s social, professional and personal life.
Emerging Hypothesis on the Initiation of Chronic Depression
The exact causes of depression are not precisely understood, but a number of factors may make depression more likely. Depression is often blamed simply on a “chemical imbalance” in the brain, but the complexities of the disease go far beyond that short statement. While research confirms that chemicals, such as neurotransmitters, are involved in depression, there are millions, even billions, of chemical reactions that occur both inside and outside of the brain’s neurons that are responsible for a person’s mood, perceptions, and how one experiences life. Depression can also be exacerbated by genetic vulnerability, stressful life events, medications and medical problems.
Current research has also indicated that depression itself is neurotoxic to the brain. Depression suppresses the levels of key neurotrophic growth factors, including fibroblast growth factor 1 (FGF-1) and brain-derived neurotrophic factor (BDNF) and this suppression leads to the eventual death of neurons found in critical memory, reasoning and mood-controlling areas of the brain, including the hippocampus and prefrontal cortex. Simply put: depression causes brain damage.
Finally, with the advent of powerful brain imaging systems, such as functional MRI, it is now possible to look at other changes in the brain that may be causative factors in major depression. What has emerged from this line of research is that a distinct reduction in blood flow or blood perfusion has been noted in those areas of the brain (hippocampus and prefrontal cortex) that appear to be the most severely affected in major depressive disorder. Thus, although a multi-factorial cause of major depression may be plausible, two major initiating events that lead to this disorder appear to be: 1) a lack of blood flow resulting in injury to the neurons involved in regulating mood, and 2) the inability of the brain to regenerate these neurons due to a suppression of critical growth factors.

Reduced Blood Flow in the Brain in Chronic Depression
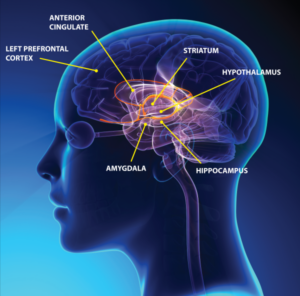
Imaging studies with functional MRI have showed a significant reduction in blood flow in patients with chronic depression. This reduction in blood flow appears to be limited to those areas of the brain involved in memory, decision making and emotions. Three main areas that suffer from under-perfusion include the hippocampus, amygdala and prefrontal cortex. As shown in Figure 1, these three areas make up the “limbic” system of the brain, a system critical in mood regulation.
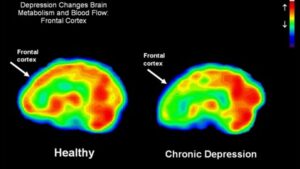
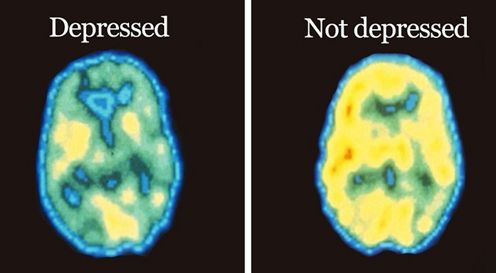
As shown in Figure 2, functional MRI scans of the brains of a healthy individual versus a patient with chronic depression reveals a dramatic decrease in in blood flow in this limbic region of the brain. Perfusion is indicated on a color scale with red/orange being normal perfusion and declining perfusion indicated by yellow and green colors. It is quite evident that the scan of the patient’s brain with chronic depression (right hand side of Figure 2) has pronounced blood perfusion deficits when compared to a healthy individual’s scan (left hand side of Figure 2) in the frontal cortex, hippocampus and amygdala areas of the brain. As has been recently appreciated with Parkinson’s disease and Alzheimer’s disease, a decline in blood perfusion seen in those disorders leads to the inevitable decline in specific populations of neurons in the brain and the subsequent devastating symptoms seen with those neurodegenerative diseases. A similar mechanism appears to be in play with chronic depression, with decreased blood perfusion of the limbic system leading to dysfunctional neurons involved in memory and mood control.
These findings suggest that a therapeutic agent that can increase blood flow in the brain may be an attractive candidate to test in patients with chronic depression. As will be discussed below, human FGF-1, with its ability to increase blood perfusion by stimulating new blood vessel growth or angiogenesis, coupled with its ability to stimulate new neuron growth (neurogenesis) may be an ideal candidate to test in patients with chronic depression.
The Role of FGF-1 in Chronic Depression
Human FGF-1 is part of the fibroblast growth factor family that consists of 22 members that display a diverse array of growth-promoting and tissue regenerating activities. The family accomplishes these biological activities by acting on a group of seven cell surface receptors that are abundant on neuronal tissues and on cells that make up the microvasculature, including vascular endothelial cells and smooth muscle cells. Human FGF-1 is the only family member that can fully activate all 7 cell surface receptors and thus is not only a potent stimulator of new blood vessel formation (angiogenesis), but can also stimulate the production of mature neurons from neural stem cells (neurogenesis).
A body of medical research has established that the FGF family, and FGF-1 in particular, can modify anxiety-like and depression-like behavior and appears to play a significant role in major depressive disorder. This concept emerged from studies of postmortem brains of subjects who had died while suffering from severe clinical depression and showed that members of the FGF family, including FGF-1, were significantly decreased in the brains of patients suffering from major depression.
To support these findings, animal studies were performed in models of depression-like behavior that more closely paralleled depression in humans. The animal models employed focused on the hippocampus, as human postmortem studies pointed to this brain region as the most altered by chronic depression.
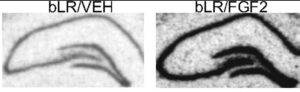
FGFs can control the development and size of the hippocampus and it has been demonstrated that FGFs and their receptors are decreased in these animal models of depression. To ascertain the possibility that FGF could act as an “endogenous antidepressant”, FGF was directly administered into the brains of depressed adult rats and multiple tests of depression-like behavior established that FGF has demonstrable antidepressant properties. In addition, it was also shown that after FGF injection into the brain, specific markers of neurogenesis were up-regulated. As shown in Figure 3, FGF injections resulted in a robust stimulation of neurogenesis as seen by the significant upregulation of a biomarker (nrk3 kinase) for neurogenesis in the gyrus dentate region of the hippocampus, an area important for memory retention and exploratory behavior in rodents.
Taken together, this body of research points to a critical role of the FGFs in mediating major depression. FGF-1, as the most potent and widely-acting member of the FGF family, and with its ability to co-regulate both angiogenesis and neurogenesis, would appear to be a good therapeutic candidate to test in patients with chronic depression.
Stress Inhibits Angiogenesis in the Hippocampus Leading to Neuronal Dysfunction
It is now well appreciated that stress plays a major role in the initiation of major depressive order and has a significant impact on the cellular integrity and function of certain brain areas, most notably the limbic structures which include the hippocampus, amygdala and prefrontal cortex (see Figure 1). These areas of the brain communicate with each other and are vital to consolidating memory, decision making and emotional responses.
The exact pathway for how stress damages neurons in these areas of the brain is unclear, but it has been proposed that stress is harmful because stress releases glucocorticoids which impair the capacity of neurons to survive. Another possibility, which has come from more recent studies, indicates that chronic stress may impair the vascular supply and damage neurons in a process very similar to traumatic cerebral vascular injury which is seen in stroke and traumatic brain injury.
Stress results in the release of glucocorticoids, such as cortisol, from the adrenal gland as part of the “fight or flight” syndrome. These glucocorticoids or corticosteroids reach the brain and immediately activate very selective areas of the brain in the limbic structures as shown in Figure 4. Using functional MRI with imaging tracers that detect metabolic activity in cells, it can be seen in Figure 4 that glucocorticoids cause a massive explosion of metabolic activity in the limbic system, including the frontal cortex and hippocampus.
Although this activation of the limbic system serves its purpose in the timely “fight or flight” decision of the individual, it has been demonstrated that repeated and constant activation of this response, as is seen in a person under constant stress, leads to pathology in these structures. The neurons become damaged by this relentless stimulation and it is a common finding in autopsies of persons who have died with chronic depression that the volumes of these structures are severely depleted.
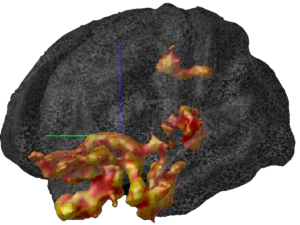
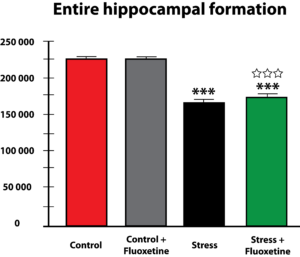
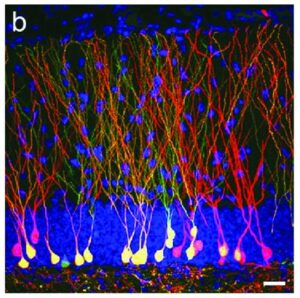
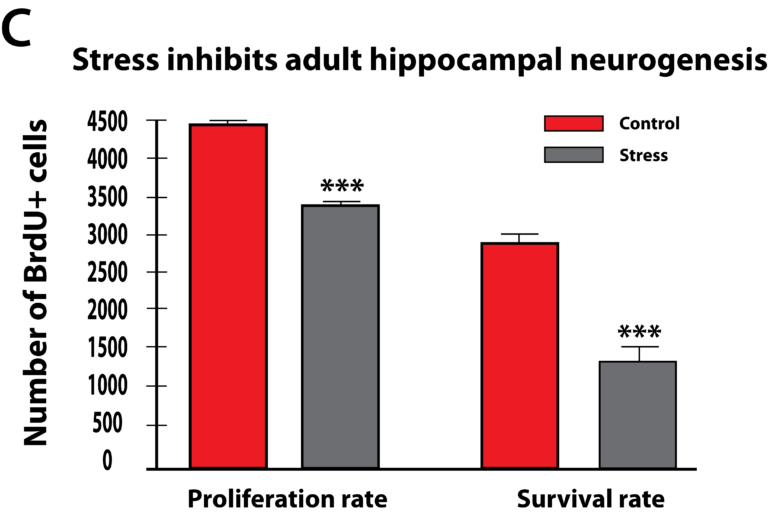
More recent studies have pointed to the fact that glucocorticoids can cause changes in vascularization, which is the major structure for supplying nutrition and oxygen to neurons in the limbic system. Glucocorticoids are potent inhibitors of angiogenesis and in an animal model of major depression, investigators showed a consistent decrease in the number of small capillaries which supply the hippocampus. These animals have a depressive disorder brought on by chronic psycho-emotional stress, which greatly elevates their serum levels of glucocorticoids. As shown in Figure 5, five weeks of daily psycho-social stress resulted in about a 30% decrease in microvessel number in all hippocampal sub-areas. Such a dramatic change is likely to have a significant impact on the oxygenation and nutritional supply of the neurons in that area of the brain.Subsequent studies in this animal model of depression established that, indeed, this decrease in capillary number also resulted in a significant decrease in neurogenesis or new neuron formation in the hippocampus. By confocal microscopy, new neuron formation and their survival could be detected and quantified in the hippocampus, as shown in Figure 6.
Thus, these and other studies clearly demonstrate that microvascular dysfunction in the brain brought about by stress and depression can have a powerful impact on the proliferation and survival of neurons in the hippocampus, an area of the brain critically responsible for proper emotional and cognitive functioning of individuals. Although most of these recent studies have focused on the hippocampus, there is no reason not to suspect that a similar mechanism may also be in play in other emotional and decision-making centers of the brain, including the amygdala and the prefrontal cortex.
An agent that can stimulate therapeutic angiogenesis, such as human FGF-1, would be an attractive candidate to test to see if it could counteract the deleterious effects of stress and stimulate new capillary growth in the hippocampus, which in turn should be coupled to enhance neurogenesis in this region of the brain which is critical for memory consolidation and mood fluctuations.
Major Depression Associated with other Diseases
A number of life-threatening diseases that affect other areas of the body often co-present with major depression. A number of such examples are given below and would represent additional targets for treatment with an agent that can promote “therapeutic angiogenesis”.
1. Heart disease (without heart attack): 15 to 20 percent experience depression
Up to 15 percent of people with cardiovascular disease and up to 20 percent of people who have had coronary artery bypass graft surgery experience major depression. In fact, patients who have had coronary artery bypass graft surgery along with untreated depression have an increased risk of dying.
Heart disease patients with depression have been shown to have increased platelet reactivity, decreased heart viability and increased proinflammatory markers — which all increase heart disease risks. Depression has become such a proven risk factor in heart disease and is so commonly missed by doctors that the American Heart Association now recommends that all cardiac patients be screened for depression.
2. Heart disease with heart attack: 40 to 65 percent experience depression
It is abundantly clear that mental stress affects our heart health, but for patients who have suffered a heart attack, depression comes into significant play. In a landmark study, researchers found that depression after recovering from a heart attack increased the risk of death to 17 percent within six months after the event, compared to 3 percent in heart attack patients who didn’t have depression.
3. Parkinson's disease: 40 percent experience depression
While being diagnosed with a chronic illness may be cause alone for depression, in the case of Parkinson's disease, there appears to be pathological causes as well. A recent brain imaging study found that people with Parkinson's disease may have an unusually high number of reuptake pumps for serotonin, the brain compound that helps regulate mood. Overactive pumps decrease serotonin levels, potentially leading to increased depression in some people with Parkinson's disease. Also, similar to the case that was made above for chronic depression, a growing number of research groups feel Parkinson’s disease is initiated by a lack of blood flow or blood perfusion of the dopamine-producing neurons, the neurons that are affected
Parkinson’s disease has similar etiologies involving the micro-vasculature.
4. Multiple sclerosis: 40 percent experience depression
Aside from the emotional stress of diagnosis, depression in patients with MS may be physically caused by the disease process itself. MS damages the myelin and nerve fibers deep within the brain — and if it affects areas that are involved in emotional processing, a number of behavioral changes can occur including the onset of depression, according to the National Multiple Sclerosis Society. Also, some drugs such as corticosteroids or interferon medications — both prescribed for MS — may trigger or deepen depression. As mentioned above, corticosteroids are also inhibitors of angiogenesis and may contribute to blood perfusion defects in patients with MS and depression.
5. Stroke: 10 to 27 percent experience depression
The National Institute of Neurological Disorders and Stroke describes post-stroke depression as, “a feeling of hopelessness that interferes with functioning and inhibits quality of life.” Left untreated, post-stroke depression can be dangerous in that it slows down recovery. Risk factors that can influence depression after stroke are both biological and behavior-influenced. Depression can stem from stroke damage to the brain as well as genetic influences, in addition to other factors including social isolation. Stroke patients suffering from depressive symptoms may be less likely to follow treatment plans, according to research reports.
6. Diabetes: 25 percent experience depression
The American Diabetes Association (ADA) warns of the problems that can happen when patients with diabetes have depression that is left untreated. The stress of managing diabetes on a day-to-day level is real, and can make people feel sad and isolated, they note. And depression and diabetes can easily spiral into a vicious cycle. “If you are depressed and have no energy, chances are you will find such tasks as regular blood sugar testing too much,” writes the ADA.
Research also shows that depression increases the risk for diabetes, and diabetes increases the risk for depression. One study found that women who were depressed were 17 percent more likely to develop diabetes, even after the scientists adjusted for other risk factors, including weight and exercise.
Conclusion
We believe that microvascular dysfunction and reduced blood perfusion in the small capillaries (the micro-vasculature) in the limbic region of the brain lead to the slow starvation of neurons there and is an important contributing factor in the development of chronic depression. Powerful new imaging techniques that can accurately quantitate the flow of blood in specific regions of the brain have documented the dramatic decrease in blood perfusion in the limbic areas of the brain in individuals suffering from chronic depression.
We also believe that “therapeutic angiogenesis” or the stimulation of new blood vessel growth by a biological drug such as the one we are developing, can be successfully utilized to overcome this lack of blood flow in patients with chronic depression. Wund is developing human growth factor, FGF-1, which is probably the most potent inducer of therapeutic angiogenesis yet discovered. Outside of the brain it has already shown great promise in US FDA Phase II clinical trials in treating a number of medical disorders characterized by a lack of blood perfusion, including coronary artery disease, diabetic foot ulcers and venous leg ulcers.
Wund’s research partner, Zhittya Genesis Medicine, has developed a “Proof of Concept” clinical trial in which FGF-1 will be tested in patients suffering from chronic depression. Three ascending doses of FGF-1 will be infused into patients five days a week for a six-week period of treatment. Safety and efficacy parameters will be measured. We believe that FGF-1 has great potential to act on the root cause of chronic depression, by stimulating both new blood vessel growth and new neuron growth, and to be a truly disease-modifying agent in reversing major depression. In addition, this therapy may also prove beneficial in treating depression that is associated with other devastating disorders, including heart disease, Parkinson’s disease, MS and stroke.
Research Papers and additional reading:
1. The FGF family: biology, pathophysiology and therapy. A Beenken and M Mohammadi. Nat Rev Drug Discov 2009; 8: 235-253. https://www.ncbi.nlm.nih.gov/pubmed/19247306
2. Cerebrovascular dysfunction with stress and depression. E Burrage, K Marshall et al. Brain Circulation 2018, 4:43-53. https://www.semanticscholar.org/paper/Cerebrovascular-dysfunction-with-stress-and-Burrage-Marshall/ac324cb3977b78fe3ee5385115c9193286a40706
3. Dysregulation of the fibroblast growth factor system in major depression. S. J. Evans, P. V. Choudary, C. R. Neal et al. Proc Natl Acad Sci (USA), 2004, 101:15506-15511. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC523463/
4. The Fibroblast Growth Factor System and Mood Disorders. C Turner, H Akil, S Watson and S Evans. Biological Psychiatry 2006, 59:1128-1135. https://www.ncbi.nlm.nih.gov/pubmed/16631131
5. Quantitative Changes in Hippocampal Microvasculature of Chronically Stressed Rats: No Effect of Fluoxetine Treatment. B Czeh, N Abumaria, R Rygula and E Fuchs. Hippocampus 2010, 20:174-185. https://www.ncbi.nlm.nih.gov/pubmed/19330847
6. The Fibroblast Growth Factor Family: Neuromodulation of Affective Behavior. C Turner, S Watson and H Akil. Neuron, 2012, 76:165-174. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3476848/
7. Subcortical brain alterations in major depressive disorder: findings from the ENIGMA Major Depressive Disorder working group. L Schmaal, D J Veltman, et al. Molecular Psychiatry volume 21, pages806–812(2016). https://www.nature.com/articles/mp201569